Great Experiments
a course at UNC Chapel Hill
How the genetic code was cracked
(chosen for Aug 31 discussion)
 |
| possible 3-letter codes (image credit) |
Supplements: Gamow's guess, Brenner disproves Gamow and all overlapping triplet codes, the decisive artificial RNA experiments
On spontaneous generation and the lack thereof (discussed by the group on 9/4)
Spontaneous generation, a widely held belief first described in the 5th century BCE, is eloquently explained by Aristotle as organisms "not derived from living parentage, but [that are] generated spontaneously: some out of dew falling on leaves, ordinarily in spring-time, but not seldom in winter…" was decidedly disproved by recent experiments of Louis Pasteur, a modern scientist of the 19th century. In a series of articles published in the 1860s, the author describes the introduction of "amianthus charged with atmospheric dust" to boiled milk or urine resulting in the growth of bacteria, including "very minute Vibriones, and Monads" leading to putrefaction. The boiled liquid when untouched remained unchanged, suggesting a substance contained on or within the dust promoted the growth of microorganisms. This simple set of experiments led to the confirmation of biogenesis (life from life) and the subsequent discovery that certain microorganisms cause disease (germ theory).
Other:
Berche, P. (2012), Louis Pasteur, from crystals of life to vaccination. Clinical Microbiology and Infection, 18: 1–6.
- Aristotle on Spontaneous Generation: Aristotle, The History of Animals, Book 5
- Pasteur on the lack thereof (English translations): Translations: On the Origin of Ferments. New experiments relative to so-termed Spontaneous Generation (1860)
- XXXIV.—A novel instance of the production of fermentation by the presence of Infusoria capable of existing without free oxygen and deprived of all access of atmospheric air (1863)
Other:
Berche, P. (2012), Louis Pasteur, from crystals of life to vaccination. Clinical Microbiology and Infection, 18: 1–6.
Chainsaw massacre in the Florida Keys - the effects of island size on species immigration and extinction rates
(chosen for Sept 28 discussion)
 |
| From Wilson and Simberloff, 1969. |
- Simberloff, D., 1976. Experimental zoogeography of islands: effects of island size. Ecology, 57 (4): 629-648.
Additional resources:
A song about the theory of island biogeography
Background information about island biogeography
Paper on the defaunation and monitoring techniques used by Wilson and Simberloff
Paper on the colonization experiments first carried out by Simberloff and Wilson
Paper on the colonization model developed by Simberloff
A 58,000-generation experiment catches evolution in the act
(chosen for Oct 26 discussion)
30 years ago, Richard Lenski started an evolution experiment that is still running today. The Long-Term Evolution Experiment, or LTEE, began in 1988 when Lenski started 12 lines of E. coli with a single clonal cell in each. By maintaining these lines under identical conditions for tens of thousands of generations and freezing still-viable samples of each line every 500 generations, Lenski created the ability to examine the repeatability of evolution and to replay evolution from any 500-generation time-point in the past. And when one line evolved a novel resource-use phenotype approximately 30,000 generations later, Lenski and colleagues were able to leverage this ingeniously simple design, using genome sequencing to reveal the genetic origin of the novel phenotype — and shedding new light on the importance of historical contingency in adaptive evolution.
A bit of model-fitting at the 60K-gen. mark suggests there may not be an upper limit to adaptation in a constant environment
A much earlier paper describing the design of the LTEE in detail
Lenski's LTEE website with more papers, data, news, and info for the lay public
An AmNat meeting talk by Lenski about where the LTEE is now, 65K gens. later
- The paper: Blount et al. 2012. Nature 489.
A bit of model-fitting at the 60K-gen. mark suggests there may not be an upper limit to adaptation in a constant environment
A much earlier paper describing the design of the LTEE in detail
Lenski's LTEE website with more papers, data, news, and info for the lay public
An AmNat meeting talk by Lenski about where the LTEE is now, 65K gens. later
Holey Moly! The history of digestion
(chosen for Nov 9 discussion)
The story of Dr. William Beaumont and a man-turned-science-experiment.
In 1822, Alexis St. Martin was accidentally shot in the stomach and treated by Dr. Beaumont. He was expected to die from his wounds, but remarkably he survived - with a hole in his stomach.
Dr. Beaumont doing what any opportunistic scientist would do, used this strange case to study digestion in the human body. For the next 11 years, St. Martin and Beaumont conducted a series of experiments ranging from the simple observation of normal digestion to the effects that temperature, exercise and even emotions have on the digestive process.
Supplement: The whole 1833 book, Experiments and observations on the gastric juice, and the physiology of digestion, by William Beaumont. Flip through it for interesting pictures and descriptions.
In 1822, Alexis St. Martin was accidentally shot in the stomach and treated by Dr. Beaumont. He was expected to die from his wounds, but remarkably he survived - with a hole in his stomach.
Dr. Beaumont doing what any opportunistic scientist would do, used this strange case to study digestion in the human body. For the next 11 years, St. Martin and Beaumont conducted a series of experiments ranging from the simple observation of normal digestion to the effects that temperature, exercise and even emotions have on the digestive process.
Supplement: The whole 1833 book, Experiments and observations on the gastric juice, and the physiology of digestion, by William Beaumont. Flip through it for interesting pictures and descriptions.
How did the blind cavefish lose its eyes?
(chosen for Nov 2 discussion)
 The Mexican tetra, Astyanax mexicanus, has been the subject of great curiosity for developmental and evolutionary biologists for decades, largely because it exists in two strikingly different forms: a surface stream-dwelling form with fully functional eyes and a cave-dwelling form that does not develop eyes and is completely blind. The surface-dwelling eyed form is ancestral, and the lack of eyes in the cave-dwelling form has inspired much speculation regarding possible selective pressures, fitness costs, and altered developmental mechanisms that might have led to the evolution of eyeless-ness. In 2013, experiments by Rohner and colleagues revealed an intriguing component of the origins of eyeless-ness in blind cavefish, serving up evidence that cryptic genetic variation masked from selection by a key developmental mechanism may have been expressed and exposed to selection upon introduction to novel stressors in the cave environment.
The Mexican tetra, Astyanax mexicanus, has been the subject of great curiosity for developmental and evolutionary biologists for decades, largely because it exists in two strikingly different forms: a surface stream-dwelling form with fully functional eyes and a cave-dwelling form that does not develop eyes and is completely blind. The surface-dwelling eyed form is ancestral, and the lack of eyes in the cave-dwelling form has inspired much speculation regarding possible selective pressures, fitness costs, and altered developmental mechanisms that might have led to the evolution of eyeless-ness. In 2013, experiments by Rohner and colleagues revealed an intriguing component of the origins of eyeless-ness in blind cavefish, serving up evidence that cryptic genetic variation masked from selection by a key developmental mechanism may have been expressed and exposed to selection upon introduction to novel stressors in the cave environment. The paper:
Do "spindle fibers" really exist in living cells?
(chosen for Sept 7 discussion)
Before 1953, the cell biology field was deeply divided in debate over the existence of apparent "fibers" in the mitotic and meiotic spindles of organisms. The only evidence for these fibers, which we know today as microtubules, came from images of fixed and stained cells. Many biologists claimed these fibers were simply artifacts of fixation, since no one had been able to see any fibrous spindle structure in any living organism with any microscope available at the time. Undeterred by the limitations of currently available technology, Shinya Inoué combined high-resolution microscopy with polarized light illumination (thus innovating polarized light microscopy) to make the invisible visible for the first time in vivo: Inoué showed spindle fibers moving with chromosomes in dividing Chaetopterus (annelid) oocytes and Lilium (lily) pollen cells. For this and his subsequent studies into these fibers, Shinya Inoué has come to be known as the father of cytoskeletal dynamics.
Related information:
1. Tribute to Shinya Inoué
2. Shinya speaking about his discovery
3. Edward Salmon (student of Shinya) on the evolution of light microscopy in the biological sciences
Related information:
1. Tribute to Shinya Inoué
2. Shinya speaking about his discovery
3. Edward Salmon (student of Shinya) on the evolution of light microscopy in the biological sciences
Primordial Soup
(chosen for Oct 5 discussion)
The origin of life is a hotly debated topic. Before the 1950s, scientists were split on the exact makeup of the Earth’s atmosphere. Some thought life originated on Earth from an environment made up of carbon dioxide, nitrogen, oxygen and water. Miller and Urey argued that life formed from the mixture of methane, ammonia, water, and hydrogen. In 1953, the pair developed a simple apparatus that could simulate their proposed hypothetical conditions, and they were able to observe that 10-15% of the carbon within the apparatus formed the building blocks of life providing evidence to their hypothesis. The results remain controversial but one cannot deny that this is a great experiment; Even Miller himself remarked once, “The fact that the experiment is so simple that a high school student can almost reproduce it is not a negative at all. The fact that it works and is so simple is what is so great about it.”
Split Brain Experiments: Two brains in one head?
(chosen for Sept 21 discussion)
In the 1960s, a treatment for epileptic patients involved the removal of the corpus callosum, a bundle of nerves that allow the two hemispheres of the brain to communicate with each other. Using these patients, Roger Sperry conducted a series of simple but ingenious experiments to demonstrate that the two hemispheres of the brain work independently to receive and react to information. For example, when an object was presented to the left hemisphere, subjects could name it. When the object was presented to the right hemisphere, subjects claimed not to have seen anything. However, they could use their left hand (which is controlled by the right brain) to draw the object. This suggested that the left brain is dominant for speech while the right brain is important for nonverbal communication. For the next 25 years Sperry further elucidated the unique functions of the left and right brains, and earned the Nobel Prize in 1981.
- Sperry RW. Hemisphere deconnection and unity in conscious awareness. AmPsychol. 1968 Oct;23(10):723-33. PubMed PMID: 5682831.
Additional information:
Keep it in your genes! Alternative splicing determines sexual orientation in flies
(chosen for Sept 14 discussion)
 |
| Modified gene splicing can alter sexual orientation in fruit flies [Image modified from Chapman and Wolfner (2017)] |
Addressing this problem in higher-level organisms presents a monumental hurdle: courtship behaviors are influenced by social expectations. To circumvent these problems, Demir and Dickson used the beautifully simple and well-characterized Drosophila melanogaster to parse out the differential effects of alternative splicing on physical and behavioral sex. Their creative experiments showed that physical sex and courtship behaviors are independently determined and that splicing of a single gene is sufficient to manipulate the inner workings of the brain.
(And here's some background on physical sex determination in Drosophila)
Selection does not induce mutations: it reveals them
(discussed Oct 12)
In the early 1900s, scientists discovered the existence of bacteria-killing viruses and soon after, observed singularities of resistant bacteria within cultures. This led to the description of bacteriophage as “dissociating agents,” yet no one could present evidence as to the mechanism of acquired resistance, what characteristic of bacteria was “dissociated” by bacteriophage. In 1943, Salvador Luria and Max Delbruck used logical deduction to simplify the problem and formulated two hypotheses: either the bacteria acquired heritable resistance in the presence of virus (adaptive) or the bacteria accrued mutations conferring resistance prior to viral exposure (spontaneous). They developed the theoretical distributions of resistant bacteria expected for each hypothesis and then experimentally observed that the actual distribution supports the mutation hypothesis. This simple logic puzzle not only answered a question in the minds of bacterial biologists; it demonstrated that mutations (beneficial or not) occur in the absence of selection. Selection simply reveals them.
Extra tidbits:
4. Six years later, Howard Newcombe published similar results in Nature.
Extra tidbits:
1. In addition to demonstrating that random, selection-independent mutations were the source of resistance in the bacterial cells, Luria and Delbruck also went so far as to formulate a mathematical model for estimating the mutation rate in a given culture. Their archaic method is one of a few models still used today.
2. The molecular basis of bacterial resistance has since been attributed to mutations in the fhuA gene, which encodes for a protein FhuA that acts as a phage receptor, among other functions. Read more here.
3. In his 1984 autobiography entitled A Slot Machine, A Broken Test Tube: An Autobiography, Salvador Luria wrote:
Everyone knows that in research there are no final answers, only insights that allow one to formulate new questions.This work initiated the field of "modern" bacterial research and with it an unending cycle of new insights and new questions.
4. Six years later, Howard Newcombe published similar results in Nature.
(image source: Wikipedia)
CRISPR-The coolest new tool for your scientific toolbox (discussed by the group on Nov 13, 2013)
- The Paper: A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity - Science 2012
Video: The CRISPR Song from iGEM-Freiberg via YouTube.
MEGA-plate: A Tool for Watching the Evolution of Antibiotic Resistance
(discussed Oct 12)
The spread of antibiotic resistance poses a very serious health threat, particularly in environments that have a high degree of antibiotic and antibacterial use, like hospitals. How bacteria evolve antibiotic resistance, particularly in spatially structured environments where we are likely to encounter them, is not well understood.
With a simple but elegant experiment, Baym et al. characterize how bacteria adapt to distinct changes in antibiotic concentration as they migrate across a massive agar plate. Their Microbial Evolution Growth Arena (MEGA)-plate provides a large enough space such that separate mutant lineages can progress without significantly impacting each other, and that spatially specific antibiotic concentrations can be maintained with minimal diffusion.
With their setup, Baym et al. were able to observe as unique mutants appeared with each progressive step in antibiotic concentration, capturing a jaw-dropping visualization of rapid evolution.
With a simple but elegant experiment, Baym et al. characterize how bacteria adapt to distinct changes in antibiotic concentration as they migrate across a massive agar plate. Their Microbial Evolution Growth Arena (MEGA)-plate provides a large enough space such that separate mutant lineages can progress without significantly impacting each other, and that spatially specific antibiotic concentrations can be maintained with minimal diffusion.
With their setup, Baym et al. were able to observe as unique mutants appeared with each progressive step in antibiotic concentration, capturing a jaw-dropping visualization of rapid evolution.
- Baym et al. Spatiotemporal microbial evolution on antibiotic landscapes, Science 2016.
Left-right patterning, situs inversus, and (artificial) nodal flow - oh my!
(discussed Oct 12)
 |
| Cilia whips its head back 'n forth to flip symmetry. [Modified from Babu and Roy, 2013; linked below] |
Studies beginning in the 1970s gave the first hint towards the solution: human patients with Kartagener syndrome had situs inversus (whereby their internal left-right axis was flipped) together with respiratory disorders linked to ciliary dysfunction. Further studies solidified the connection between ciliary motion and left-right axis specification, but it was not until the cleverly-designed experiments of Nonaka et al. in 2002 that cilia-induced nodal flow was shown to be sufficient for left-right patterning in developing mouse embryos.
And here's a wonderful review on L-R patterning, for those interested.
Using Frankenstein-like rats to study the hypothalamus
(discussed Oct 12)
 Parabiosis is a technique that involves surgically joining two living organisms such that they share a circulatory system. In 1959, G. R. Hervey utilized parabiosis to study the role of the brain’s hypothalamus in obesity. Hervey combined pairs of rats in which one rat had a surgical lesion in the hypothalamus and the other rat was healthy. Hervey noticed that the lesioned rat became obese and experienced significant weight gain and excessive hunger, while the healthy rat experienced weight loss and decreased appetite. The results suggested that there exists a feedback control system in the hypothalamus involving physiological signals that are released in order to suppress appetite. The healthy rat had decreased its eating in response to the signals in the blood from the lesioned rat whose feedback control system was impaired. In addition to obesity, parabiotic experiments have been used to study age-related chronic diseases (e.g. Alzheimer’s and osteoarthritis), stem cells, tissue regeneration, diabetes, and cancer among others.
Parabiosis is a technique that involves surgically joining two living organisms such that they share a circulatory system. In 1959, G. R. Hervey utilized parabiosis to study the role of the brain’s hypothalamus in obesity. Hervey combined pairs of rats in which one rat had a surgical lesion in the hypothalamus and the other rat was healthy. Hervey noticed that the lesioned rat became obese and experienced significant weight gain and excessive hunger, while the healthy rat experienced weight loss and decreased appetite. The results suggested that there exists a feedback control system in the hypothalamus involving physiological signals that are released in order to suppress appetite. The healthy rat had decreased its eating in response to the signals in the blood from the lesioned rat whose feedback control system was impaired. In addition to obesity, parabiotic experiments have been used to study age-related chronic diseases (e.g. Alzheimer’s and osteoarthritis), stem cells, tissue regeneration, diabetes, and cancer among others.
The Method of DNA Replication
(discussed Oct 12)
 Three models of DNA replication were popular when Watson and Crick discovered the structure of DNA in 1953: conservative, semi-conservative, and dispersive. The conservative model proposed an entirely new DNA double helix was synthesized during each round of replication, resulting in one "new" helix and one "old" helix. According to semi-conservative model, each round of replication results in hybrid helices with one new strand and one old strand. The dispersive model also suggested hybrid DNA molecules, but the pieces were randomly dispersed throughout the helices. It took until 1958 for Meselson and Stahl to identify the semi-conservative model as the correct model. They measured the density of DNA molecules after subsequent replications when E. coli was transferred from N15 containing media to N14 media. This elegantly simple experiment laid the foundation for the discovery of many of the enzymatic processes involving DNA.
Three models of DNA replication were popular when Watson and Crick discovered the structure of DNA in 1953: conservative, semi-conservative, and dispersive. The conservative model proposed an entirely new DNA double helix was synthesized during each round of replication, resulting in one "new" helix and one "old" helix. According to semi-conservative model, each round of replication results in hybrid helices with one new strand and one old strand. The dispersive model also suggested hybrid DNA molecules, but the pieces were randomly dispersed throughout the helices. It took until 1958 for Meselson and Stahl to identify the semi-conservative model as the correct model. They measured the density of DNA molecules after subsequent replications when E. coli was transferred from N15 containing media to N14 media. This elegantly simple experiment laid the foundation for the discovery of many of the enzymatic processes involving DNA.image credit: Pray, Nature Education, 2008
What do platform shoes and the overthrowing of the Berlin wall have in common?
(discussed Oct 12)
Early 1960s. Suzie is trying on platform shoes. "I really like them but people will think I look ridiculous. Well heck, I don't care what others think. I like them!"
Berlin, November 9th, 1989. Franz is standing in front of the wall hammer in hand. He heard the news on the radio, the border just opened. "Time to tear down that wall!" he thought. At first, it was only him chipping away at the wall, but the crowd soon grew bigger and bigger.
The spread of certain behaviors relies heavily on individual thresholds. How many people have to wear platform shoes or hammer away at the wall before I join them? Is my threshold low like Franz and Suzie's? In 1978, Granovetter shows how very similar crowds can react completely differently because of individual thresholds. The applications of his model are endless: from fashion crazes to riots and voting behavior.
A great video explaining this model (from the Model Thinking course on Coursera, which I highly recommend).
Berlin, November 9th, 1989. Franz is standing in front of the wall hammer in hand. He heard the news on the radio, the border just opened. "Time to tear down that wall!" he thought. At first, it was only him chipping away at the wall, but the crowd soon grew bigger and bigger.
The spread of certain behaviors relies heavily on individual thresholds. How many people have to wear platform shoes or hammer away at the wall before I join them? Is my threshold low like Franz and Suzie's? In 1978, Granovetter shows how very similar crowds can react completely differently because of individual thresholds. The applications of his model are endless: from fashion crazes to riots and voting behavior.
- Granovetter, M. S., 1978. Threshold models of collective behavior. American Journal of Sociology, 83 (6): 1420-1443.
A great video explaining this model (from the Model Thinking course on Coursera, which I highly recommend).
Would you drink a broth of bacteria to prove the real case of an illness?
(discussed Oct 12)
 By the 1970s, cases of people with gastric ulcers were recurrent. Stress, spicy food, and lifestyle were thought to provoke peptic ulcers because no one believed in the presence of bacteria in the stomach. In 1981, Barry Marshall and Warren performed biopsies on patients and were able to isolate an unknown bacterial species present in almost all patients with gastric infarction, duodenal ulcers or gastric ulcers. For this reason, they proposed that this unknown bacterium was the cause of the disease. Their experiments and discoveries were not immediately accepted. Due to prohibitions on human subjects and after trying different animal models with no success, Marshall underwent a gastric biopsy to demonstrate absence of that bacterium and then swallowed bacterial broth that possessed the bacteria of an infected patient. After several days, the disease developed and the second biopsy of his own intestine proved that in effect, the bacteria was the cause of the ulcer.
By the 1970s, cases of people with gastric ulcers were recurrent. Stress, spicy food, and lifestyle were thought to provoke peptic ulcers because no one believed in the presence of bacteria in the stomach. In 1981, Barry Marshall and Warren performed biopsies on patients and were able to isolate an unknown bacterial species present in almost all patients with gastric infarction, duodenal ulcers or gastric ulcers. For this reason, they proposed that this unknown bacterium was the cause of the disease. Their experiments and discoveries were not immediately accepted. Due to prohibitions on human subjects and after trying different animal models with no success, Marshall underwent a gastric biopsy to demonstrate absence of that bacterium and then swallowed bacterial broth that possessed the bacteria of an infected patient. After several days, the disease developed and the second biopsy of his own intestine proved that in effect, the bacteria was the cause of the ulcer.
For your information: https://www.youtube.com/watch?v=AO1T-NhcVoU&feature=youtu.be
Genotype after phenotype? Waddington and the "Genetic Assimilation of an Acquired Character"
(discussed Oct 12)
 Can an apparently "acquired" character, initially induced by environmental perturbation in the course of development, become a genetically induced trait in a population? Using a series of Drosophila selection experiments, Conrad Waddington answered this question in the affirmative. By selecting for a heat-shock-induced aberrant wing phenotype, Waddington produced lines that continued to develop the aberrant phenotype in subsequent generations even in the absence of the once-necessary environmental stimulus. The founding population did not express this phenotype without the heat-shock treatment, but after continuing to breed together only those adults that expressed the phenotype in the absence of heat shock, Waddington eventually produced some lines in which 100% of offspring expressed the phenotype without being exposed to heat shock. This is the seminal work that inspired far greater understanding and greater interest in the origins of phenotypic novelty and variation and their importance to adaptive evolution.
Can an apparently "acquired" character, initially induced by environmental perturbation in the course of development, become a genetically induced trait in a population? Using a series of Drosophila selection experiments, Conrad Waddington answered this question in the affirmative. By selecting for a heat-shock-induced aberrant wing phenotype, Waddington produced lines that continued to develop the aberrant phenotype in subsequent generations even in the absence of the once-necessary environmental stimulus. The founding population did not express this phenotype without the heat-shock treatment, but after continuing to breed together only those adults that expressed the phenotype in the absence of heat shock, Waddington eventually produced some lines in which 100% of offspring expressed the phenotype without being exposed to heat shock. This is the seminal work that inspired far greater understanding and greater interest in the origins of phenotypic novelty and variation and their importance to adaptive evolution.- The paper: C.H. Waddington 1953. Evolution 7.
1. Another genetic assimilation paper, in which Waddington expresses his thoughts on the potential evolutionary importance of the phenomenon.
2. Mary Jane West-Eberhard synthesizing her theory of plasticity-first evolution.
3. A recent TREE article by UNC researchers about plasticity-first evolution.
FrankenCells: how fusing cells together led to an understanding of cell cycle regulation
(discussed Oct 12)
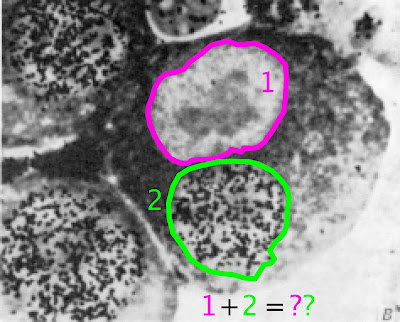 |
| image adapted from Rao & Johnson, 1970 (Nature) |
In the 1970s and 80s, the cell cycle was HOT. Major outstanding questions remained unanswered: namely how do cells "know" when to duplicate their DNA and divide? The molecular answers to these questions would come later in the decade from mutational experiments, but Rao and Johnson (1970) used a more clever approach: fusing cells in different parts of the cell cycle together and observe the effects on each nucleus's chromatin. If each cell cycle phase (G1, S, G2, M) uses different signaling factors in its cytoplasm, then what happens when those signals mix? Rao and Johnson showed not only that multinucleate cells will synchronize their nuclear division cycles, but that certain phases in the cell cycle can "dominate" over other phases. The authors' ingenuity required no great advances in genetic or optical tools; rather, they used a well-known virus to simply fuse together large populations of cells.
Can fearful memories be passed down generations?
(discussed Oct 12)
 In this paper, mice whose father or grandfather learned to associate the smell of cherry blossom with an electric shock became more jumpy when smelling the same scent. They even responded to lower concentrations of it than normal mice (whose fathers weren't exposed). Many studies hint that stress or other events can change the immune system, emotional response, or metabolic health of future generations through epigenetic inheritance.
In this paper, mice whose father or grandfather learned to associate the smell of cherry blossom with an electric shock became more jumpy when smelling the same scent. They even responded to lower concentrations of it than normal mice (whose fathers weren't exposed). Many studies hint that stress or other events can change the immune system, emotional response, or metabolic health of future generations through epigenetic inheritance.Brian Dias and his lab at Emory University School of Medicine in Atlanta, provide some of the best evidence yet for the inheritance of memories or traits across generations, as well as providing potential biological mechanisms by which this phenomenon is happening.
Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling
(discussed Oct 12)
How do cells move? The ability of living cells to crawl around has interested scientists for generations, and it has been known for a long time that actin dynamics play a critical role in allowing cells to move. In vitro work showed that actin was capable of treadmilling, but a role for treadmilling in vivo was not shown until 1985, when Wang injected fluorescently labeled actin into living fibroblast cells and visualized the flow of subunits from the periphery of the lamellapodium to the cell center by photobleaching a region at the edge of the cell and watching the bleached region move backwards. From these simple FRAP experiments he was able to infer a great deal about the dynamics of actin filaments and how they could contribute to cell crawling.
How cats learn to see
In the late 50’s and early 60’s, Hubel and Weisel pioneered the understanding of visual circuitry, showing how spots of light can be transformed through different cell types in the brain into recognition of lines, orientation, and movement. A major component of this system, “complex cells,” respond to lines of very specific orientations that cover 360 degrees, or “go around the clock”. However, it remained unclear whether this circuitry was hard-wired from birth, or could be altered by experiences in development. In a series of clever experiments, Blakemore and Cooper raised kittens from birth in rooms containing only horizontal or vertical lines.
When the cats were removed from these conditions, they continued to have developmental defects. For instance, cats raised in the horizontal condition would bump into table legs. When they examined the responses of these “complex cells” to lines of different orientations in the vertical and horizontal cats they found that the complex cells only responded to lines of a given orientation. This showed that visual experiences are developmentally dependent and was a major experiment showing that there are critical periods of brain development.
Background:
Hubel and Wiesel Original Experiments
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1363130/pdf/jphysiol01298-0128.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1359523/pdf/jphysiol01247-0121.pdf
When the cats were removed from these conditions, they continued to have developmental defects. For instance, cats raised in the horizontal condition would bump into table legs. When they examined the responses of these “complex cells” to lines of different orientations in the vertical and horizontal cats they found that the complex cells only responded to lines of a given orientation. This showed that visual experiences are developmentally dependent and was a major experiment showing that there are critical periods of brain development.
Background:
Hubel and Wiesel Original Experiments
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1363130/pdf/jphysiol01298-0128.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1359523/pdf/jphysiol01247-0121.pdf
Daddy, can I has a pet fox please?
"Njet! Now go clean up your room." For most kids in Russia, this would have been the standard answer to their
wishful request to own a pet fox. Unless their dad was the famous soviet geneticist Dmitry Belyaev. In 1959, Belyaev founded the Institute of
Cytology and Genetics in Siberia and started a lifelong experiment to
domesticate silver-black foxes. By selecting only for behavior, he
obtained a tame fox population that started to show morphological and
physiological changes not selected for but also present in many other
domesticated species. These changes, including instances of
pedomorphosis, suggest that behavioral traits favorable to domestication
are genetically linked to morphological and physiological traits. So
when his children asked him for a pet fox, Belyaev's answer might have
been "Why, yes, of course! Just give me a couple of decades. And you
must not forget: you become responsible, forever, for what you have
tamed."
- Trut, L. N., 1999. Early canid domestication: the farm-fox experiment. American Scientist, 87 (2): 160-169.
Prismatic coloration: how Newton showed different colors of light behave differently
 |
| Image credit: The Dark Side of the Moon, Pink Floyd, 1973 |
Other readings:
Morgan's Fly Room
The Fly Room at Columbia University - Restored to Its Original State from Imaginal Disc on Vimeo
Since this is my last post, and there won't be any more voting, I thought I'd share a very cool project that I recently came across that recreated Thomas Hunt Morgan's famous fly room at Columbia University. The fly room was a hot, cramped, putrid smelling room where Morgan and his colleagues unraveled some of the biggest mysteries of heredity and sex determination, work that earned them the Nobel Prize in 1933.
The room was recreated for a 2014 feature film aptly named "The Fly Room," and they do a remarkable job of recreating the details of the lab. The production company, Imaginal Disc, also put together a great website with some stories about the lab and interviews with Morgan's daughter Betsy. The video above is a short produced by Imaginal Disc showing off the room, and I've linked to the trailer for the full movie below.
Thanks everyone for a great seminar!
Imaginal Disc: http://www.imaginaldisc.com/
Trailer: https://www.youtube.com/watch?v=Cejz9HKRS-M
The Fly Room website: http://www.theflyroom.com/
Since this is my last post, and there won't be any more voting, I thought I'd share a very cool project that I recently came across that recreated Thomas Hunt Morgan's famous fly room at Columbia University. The fly room was a hot, cramped, putrid smelling room where Morgan and his colleagues unraveled some of the biggest mysteries of heredity and sex determination, work that earned them the Nobel Prize in 1933.
The room was recreated for a 2014 feature film aptly named "The Fly Room," and they do a remarkable job of recreating the details of the lab. The production company, Imaginal Disc, also put together a great website with some stories about the lab and interviews with Morgan's daughter Betsy. The video above is a short produced by Imaginal Disc showing off the room, and I've linked to the trailer for the full movie below.
Thanks everyone for a great seminar!
Imaginal Disc: http://www.imaginaldisc.com/
Trailer: https://www.youtube.com/watch?v=Cejz9HKRS-M
The Fly Room website: http://www.theflyroom.com/
The "Hunt" for Cyclin
In the early 1980s, much was known about hairspray, big hair, and questionable fashion. However, in the world of cell biology, little was know how exactly the process that allowed cells to divide was regulated. Enter Tim Hunt and a lab full of eager Physiology students that spent their summer radio labeling sea urchin eggs that they had collected from the nearby sea. They noticed something strange on their protein gels, a protein (later to be named cyclin) would accumulate and then disappear at the onset of cell division. This discovery would set into motion thousands of experiments and one Nobel Prize that led to the discovery of the fundamental mechanism of cell division. This discovery has had far reaching implications in the fields of developmental, cell, and cancer biology to name a few. This classic experiment, although simple, was cleverly designed and allowed the capture of one of the most studied processes in all of science.
Can exosomal microRNAs control insulin sensitivity in vivo?
The mechanism is a little complicated, but what if we could treat Type 2 Diabetes or insulin sensitivity with a simple injection of small non-coding RNAs? (Specifically microRNAs)
In this paper, they use a mouse model to study obesity, insulin sensitivity and exosomal RNA. Basically, they inject extracellular vesicles, or exosomes, from a lean mouse into an obese mouse with insulin sensitivity problems and the impairments are reversed. Exosomes from the obese mice into the lean mice cause insulin resistance. Will this also work as a potential future therapeutic for humans?
In this paper, they use a mouse model to study obesity, insulin sensitivity and exosomal RNA. Basically, they inject extracellular vesicles, or exosomes, from a lean mouse into an obese mouse with insulin sensitivity problems and the impairments are reversed. Exosomes from the obese mice into the lean mice cause insulin resistance. Will this also work as a potential future therapeutic for humans?
- http://www.cell.com/cell/fulltext/S0092-8674(17)30993-5 <-- The paper
DNA Damage Checkpoint is Rad!!!
How are cells able to replicate themselves so precisely, again and again? Going through each division cycle requires that the cell coordinate many complicated processes, and yet they manage to do it with extremely high accuracy and precision each time. But how does the cell make sure that everything happens in the correct order, and that any mistakes are corrected before going to the next step?
For example, how do cells ensure that DNA is accurately replicated before the cell goes through mitosis? In the 1980’s, Weinert and Hartwell reasoned there are two simple possibilities- first, successful mitosis could directly depend on having properly replicated chromosomes, rather like a car needing wheels in order to move. The other possibility is that the cell has a control system in place that halts the cell in S phase until it senses that the DNA is correctly replicated, similar to an inspector examining the car before it can leave the shop.
But how to distinguish between these two possibilities? The investigators looked specifically for mutant cells that did NOT arrest in response to radiation, indicating that this hypothetical control system was no longer functioning. These cells were missing their DNA damage checkpoint, and could no longer detect and repair damaged DNA- a critical step in ensuring faithful replication!
For example, how do cells ensure that DNA is accurately replicated before the cell goes through mitosis? In the 1980’s, Weinert and Hartwell reasoned there are two simple possibilities- first, successful mitosis could directly depend on having properly replicated chromosomes, rather like a car needing wheels in order to move. The other possibility is that the cell has a control system in place that halts the cell in S phase until it senses that the DNA is correctly replicated, similar to an inspector examining the car before it can leave the shop.
But how to distinguish between these two possibilities? The investigators looked specifically for mutant cells that did NOT arrest in response to radiation, indicating that this hypothetical control system was no longer functioning. These cells were missing their DNA damage checkpoint, and could no longer detect and repair damaged DNA- a critical step in ensuring faithful replication!
DYING GOATS, CANNIBALISM, And the central dogma
When 18,000 sheep in Scotland came down with a fatal neurodegenerative disease after receiving vaccinations, researchers were puzzled. The mystery became even greater when it was discovered that this disease, scrapie, was transmissible, and the infectious agent was in the formalin treated inoculum. After all, formalin should inactivate any infectious agent, since they all contain nucleic acids. This idea, that nucleic acids are the only mechanism by which heritable information is replicated, is known as the “central dogma” of biology.
So if 18,000 sheep dying of the mystery illness, scrapie, isn’t weird enough, let’s add in a cannibalistic tribe on a small island in the Pacific. The Fore people were dying of a strikingly similar illness, and it appeared to be transmitted through consumption of human flesh. The brains of affected people bore the same hole-ridden, spongey texture seen in biopsies of scrapie-affected sheep. What agent could be responsible for two infectious, neurodegenerative diseases? How could it be resistant to formalin?
Many hypotheses were offered, ranging from “slow virus” to “replicating polysaccharide”. However, it was work by Stanley Prusiner that lead to the unravelling of this mystery. Through tried and true reductionist biochemistry, Prusiner achieved the purest preparation of the scrapie agent yet. After quantitatively determining the amount of infectivity present in his preparation, he sought to determine what treatments would eliminate that infectivity. Through simple biochemical experiments, such as treatments with proteases and nucleases, Prusiner demonstrated that the scrapie agent was predominantly proteinaceous in nature. Prusiner also calculated the size of the agent, less than 50,000 Daltons, and inferred that this size did not allow for the presence of a replicating nucleic acid within the structure. And thus the term prion, or proteinaceous infectious agent, was coined by Prusiner. For his ability to follow his data and think beyond the central dogma, Prusiner was awarded the Nobel Prize, only 15 years later.
So if 18,000 sheep dying of the mystery illness, scrapie, isn’t weird enough, let’s add in a cannibalistic tribe on a small island in the Pacific. The Fore people were dying of a strikingly similar illness, and it appeared to be transmitted through consumption of human flesh. The brains of affected people bore the same hole-ridden, spongey texture seen in biopsies of scrapie-affected sheep. What agent could be responsible for two infectious, neurodegenerative diseases? How could it be resistant to formalin?
Many hypotheses were offered, ranging from “slow virus” to “replicating polysaccharide”. However, it was work by Stanley Prusiner that lead to the unravelling of this mystery. Through tried and true reductionist biochemistry, Prusiner achieved the purest preparation of the scrapie agent yet. After quantitatively determining the amount of infectivity present in his preparation, he sought to determine what treatments would eliminate that infectivity. Through simple biochemical experiments, such as treatments with proteases and nucleases, Prusiner demonstrated that the scrapie agent was predominantly proteinaceous in nature. Prusiner also calculated the size of the agent, less than 50,000 Daltons, and inferred that this size did not allow for the presence of a replicating nucleic acid within the structure. And thus the term prion, or proteinaceous infectious agent, was coined by Prusiner. For his ability to follow his data and think beyond the central dogma, Prusiner was awarded the Nobel Prize, only 15 years later.
The Unknown Vital Ingredient
At the turn of the 20th century, diseases such as scurvy and beri-beri were thought to be caused by microbial infections. However, experiments by Eijkman and others found dietary supplementation could cure these diseases. Eijkman then proposed that improperly prepared foods contained disease causing toxins. This theory was disproved by studies showing food contained an active factor vital for health, rather than a toxin. To try and identify the active factor, Gowland Hopkins fed rats a diet of purified protein, fat, and carbohydrates: the only known essential components of food. He found the rats ceased to grow unless their diet was supplemented with milk. Therefore, food must contain some other essential nutrient. Over the next few decades, other labs identified the essential nutrients we now know as vitamins. Although, the term vitamin was coined in 1910 when Casimir Funk falsely believed he had isolated a vital amine as the "active factor."
Other readings:
The Nobel Prize and the Discovery of Vitamins https://pdfs.semanticscholar.org/26b3/ac4d28c9bde1cbd840d5e1d17dea0a2116a3.pdf
Evolutionary family planning: life-history experiments in a natural population
 What determines the timing and characteristics of major life events, such as sexual maturation, reproductive investment, and the onset of senescence? Because these types of traits, or life-history parameters, are key determinants of fitness, evolutionary theory has long proposed that, to the extent that these traits are heritable, they should manifest as adaptations to the particular environments and ecologies of natural populations. Further, changes in a natural population's environment should result in life-history evolution whenever altered timing or qualities of life-history parameters can increase fitness. Reznick et al. set out in the 1970s to test these predictions in a natural fish population by transplanting a subpopulation to a section of stream with a very different predation regime, one in which changes in the timing of maturation and reproduction should have been adaptive. Their 11-year field experiment provided the first solid experimental evidence of life-history evolution in nature.
What determines the timing and characteristics of major life events, such as sexual maturation, reproductive investment, and the onset of senescence? Because these types of traits, or life-history parameters, are key determinants of fitness, evolutionary theory has long proposed that, to the extent that these traits are heritable, they should manifest as adaptations to the particular environments and ecologies of natural populations. Further, changes in a natural population's environment should result in life-history evolution whenever altered timing or qualities of life-history parameters can increase fitness. Reznick et al. set out in the 1970s to test these predictions in a natural fish population by transplanting a subpopulation to a section of stream with a very different predation regime, one in which changes in the timing of maturation and reproduction should have been adaptive. Their 11-year field experiment provided the first solid experimental evidence of life-history evolution in nature. 
The paper:
More:
Another great story of evolution in this system: Endler 1982
Reznick lab website









